22 July 2023 | Healthcare
The Rise of Humira Biosimilars and the Adoption Challenge
By workweek
AbbVie’s Humira—the most financially successful drug in history—said goodbye to two decades of U.S. market exclusivity back in January when Amgen’s Humira biosimilar, Amjevita, hit the U.S. market. Now, seven months later, several new Humira biosimilars are hitting the U.S. market.
In this article, I’ll highlight how several drugmakers broke through Humira’s patent fortress, discuss the latest Humira biosimilars to hit the market this month, and add my thoughts on the whole “biosimilar” market.
The Deets
AbbVie’s success over the past 20 years is credited to the patent fortress the company built around Humira, extending the drug’s patent from 2016 (expiration of the original patent) to 2034. Maybe “fortress” is an understatement. AbbVie filed over 130 patents during the past two decades.
It was all legal, too.
This patent fortress made it impossible for Humira’s competitors to enter the market. A competitor would have needed to challenge several of Humira’s 130+ patents in court—an egregiously expensive and time-consuming process. The easiest option for Humira’s competitors has been to not compete. You can read my previous recap here.
Breaking through the fortress
AbbVie settled with several U.S. drugmakers to allow Humira biosimilars’ entry into the U.S. market in 2023. The catch? These drugmakers will pay AbbVie royalties on the net sales of their Humira biosimilar based on the individually held patents.
Amgen’s Amjevita was the first Humira biosimilar to hit the market in January, and now several others have launched this month. Many of these biosimilars released a high-list price and low-list price form of the drug (more details in my dissection below). Large PBMs like Express Scripts, OptumRx, and Caremark have all added the high-list price version of Amjevita to their formularies.
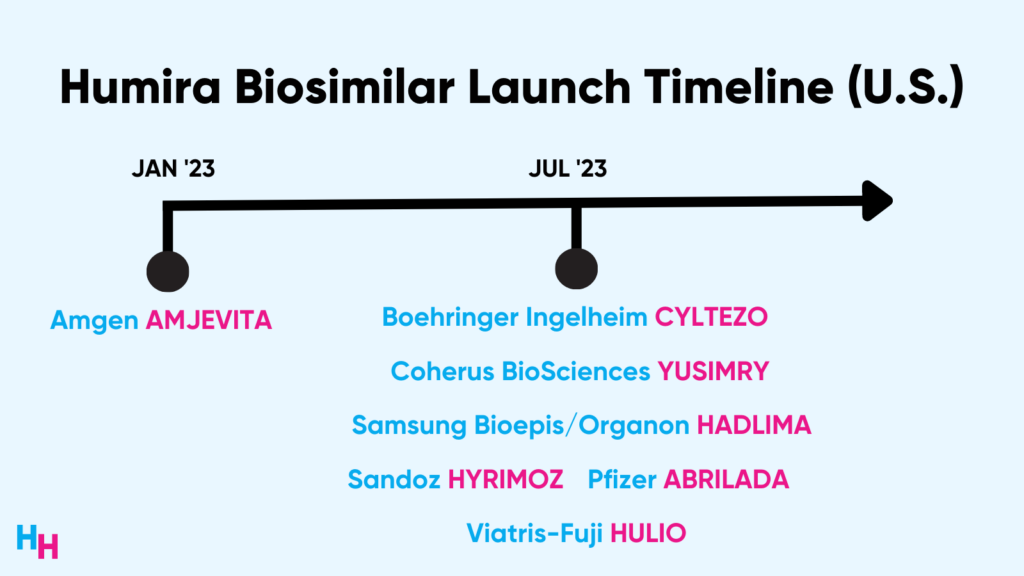
In June, OptumRx announced it would add two other Humira biosimilars, Cyltezo and Hyrimoz, to its formulary at parity with Humira. Cyltezo is an interchangeable biosimilar (pharmacists can replace Humira with Cyltezo without physician authorization) and has a list price 5% to 7% below Humira’s. While Hyrimoz isn’t an interchangeable, its list price is also 5% below Humira’s. So still high list prices!
This month, Express Scripts announced it would also include Cyltezo, Hyrimoz, and unbranded adalimumab-adaz on their National Preferred Formulary. But, the unbranded adalimumab-adaz will be listed at an 81% discount compared to Humira’s list price.
So you see this trend of PBMs only including the high-list price versions of some of the Humira biosimilars. Look at the table below from BR&R to see the list-price discounts (”WAC Discount”).
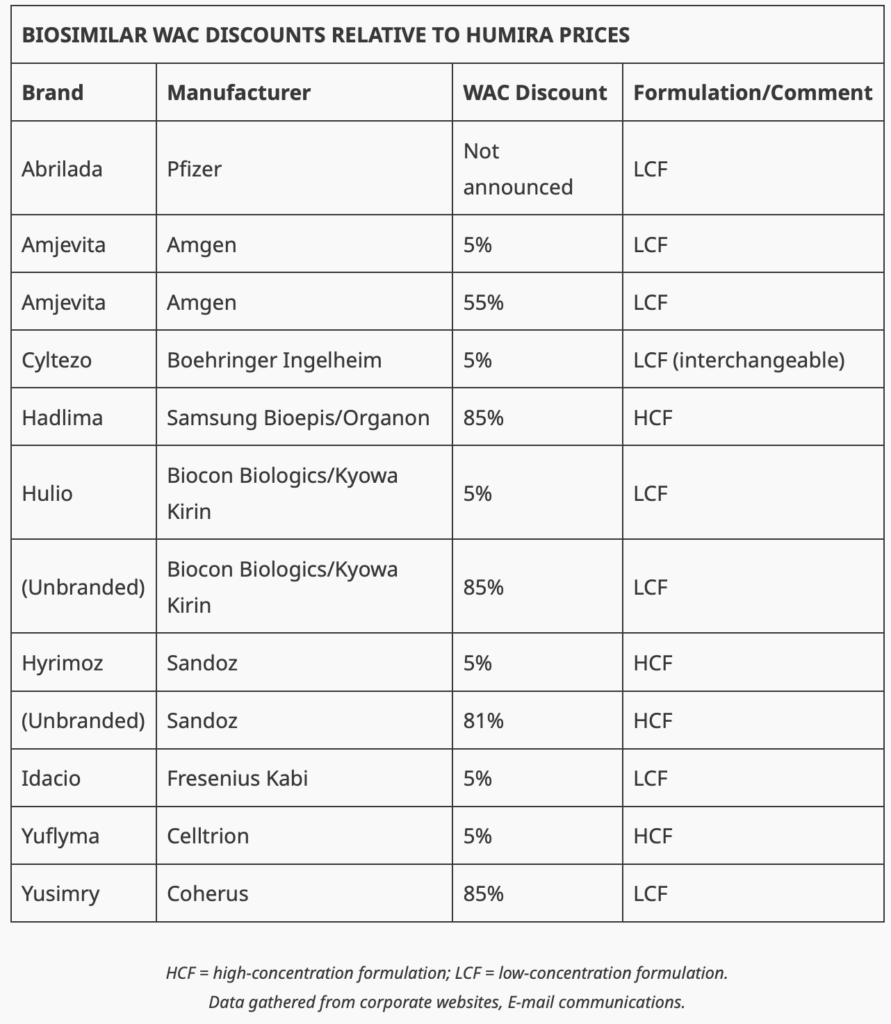
Notably, and perhaps not surprisingly, Mark Cuban’s Cost Plus Drugs announced it’s partnering with drugmaker Coherus to make Humira biosimilar Yusimry. Check the list above. What’s the discount you see for Yusimry? 85%! Cost Plus Drugs’ PBM partners, like RxPreferred, will have Yusimry on their formularies—again, an 85% discount from Humira’s list price. (I recently recapped all Cost Plus Drugs has accomplished in the first half of the year—quite remarkable! Read here).
Dash’s Dissection
Here’s the one point I want to drive home:
- Just because a drugmaker offers its Humira biosimilar at a steep discount compared to Humira, it does not mean PBMs will jump to include it on the formularies that they prepare for health insurers.
To understand why, you need to understand how the drug supply chain works.
Drug manufacturers pass rebates—a dollar amount that’s a percentage of the drug’s list price— to pharmacy benefit managers. PBMs give a portion of these rebates to the insurer, who then passes a bit to employers. Rebates are the “shady” area of the drug supply chain, and PBMs make a lot of money from rebates. So, for the Humira biosimilar to remain competitive with Humira, its list price needs to be close to Humira’s so PBMs can receive high rebates.
- PBMs are incentivized to maintain costly drugs on their formularies and forgo “cheaper” ones.
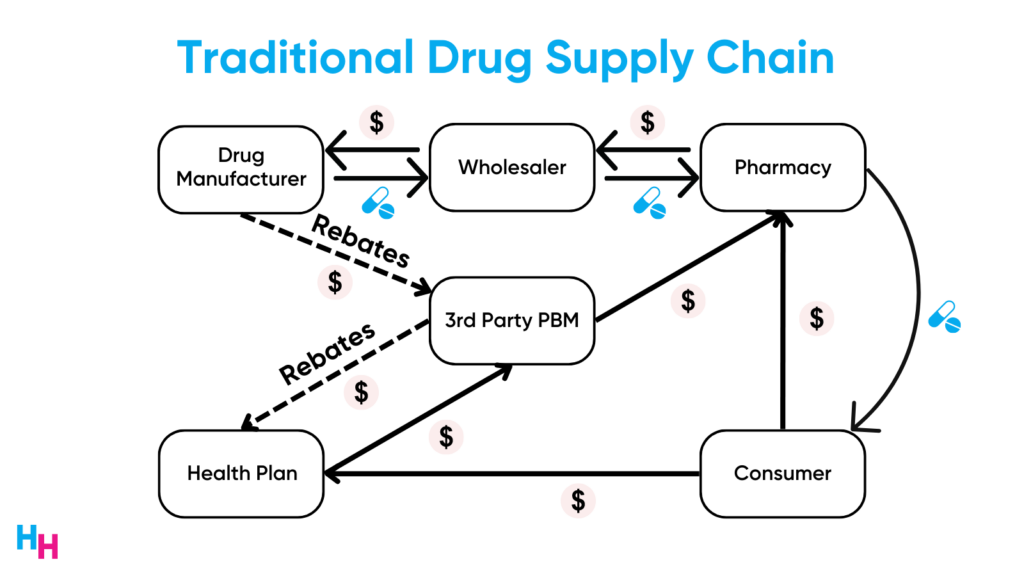
Need proof? OptumRx’s Premium Formulary excluded the low-price version of Amjevita but kept the high-price version. On another formulary, OptumRx’s Select Formulary, the low-price version wasn’t excluded, but was ranked Tier 3 (low). Patients are required to first try Humira and the high-price version before accessing the low-price version. This healthcare system is funny, isn’t it?
But what about Cost Plus Drugs? How are they getting away with including a Humira biosimilar that’s 85% cheaper than Humira’s list price?
Cost Plus Drugs bypass PBMs, so they don’t have to worry about the rebates PBMs need to stay afloat. Keep in mind though, that because Cost Plus Drugs (for the most part) doesn’t deal with insurance, the ~$500 price of Yusimry is out-of-pocket.
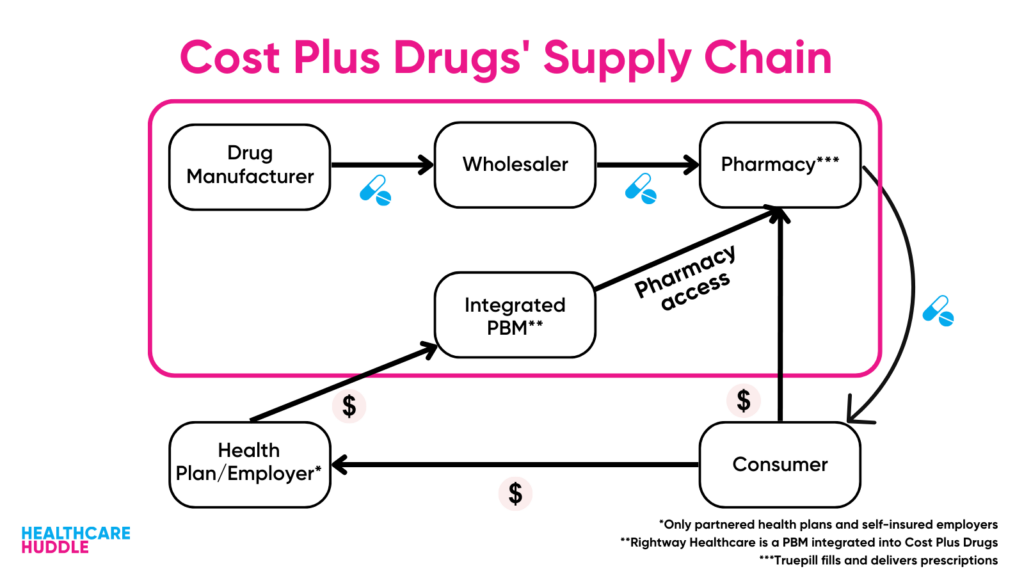
However, Cost Plus Drugs does partner with some PBMs who work with employers and insurers. These PBMs values are perfectly aligned with Cost Plus Drugs’ values of price transparency. This is why RxPreferred—one of the partnered PBMs—is including Yusimry on their formulary. Without a doubt, we’ll see Cost Plus Drugs continue to get into the biosimilars market, choosing to add the cheapest biosimilar on their formulary.
In summary, multiple Humira biosimilars are entering the market, challenging AbbVie’s patent fortress. However, the complex drug supply chain and the influence of pharmacy benefit managers (PBMs) present hurdles for biosimilar adoption. Some companies, like Cost Plus Drugs, bypass PBMs and offer significantly cheaper options. Overall, the biosimilar market for Humira is evolving, but challenges persist for widespread PBM and insurer acceptance.
Stay ahead in healthcare with my weekly Healthcare Huddle newsletter, covering digital health, policy, and business trends for 30,000+ professionals. Share with colleagues and subscribe here.